5 Tips about modafinil mechanism of action You Can Use Today
5 Tips about modafinil mechanism of action You Can Use Today
Blog Article
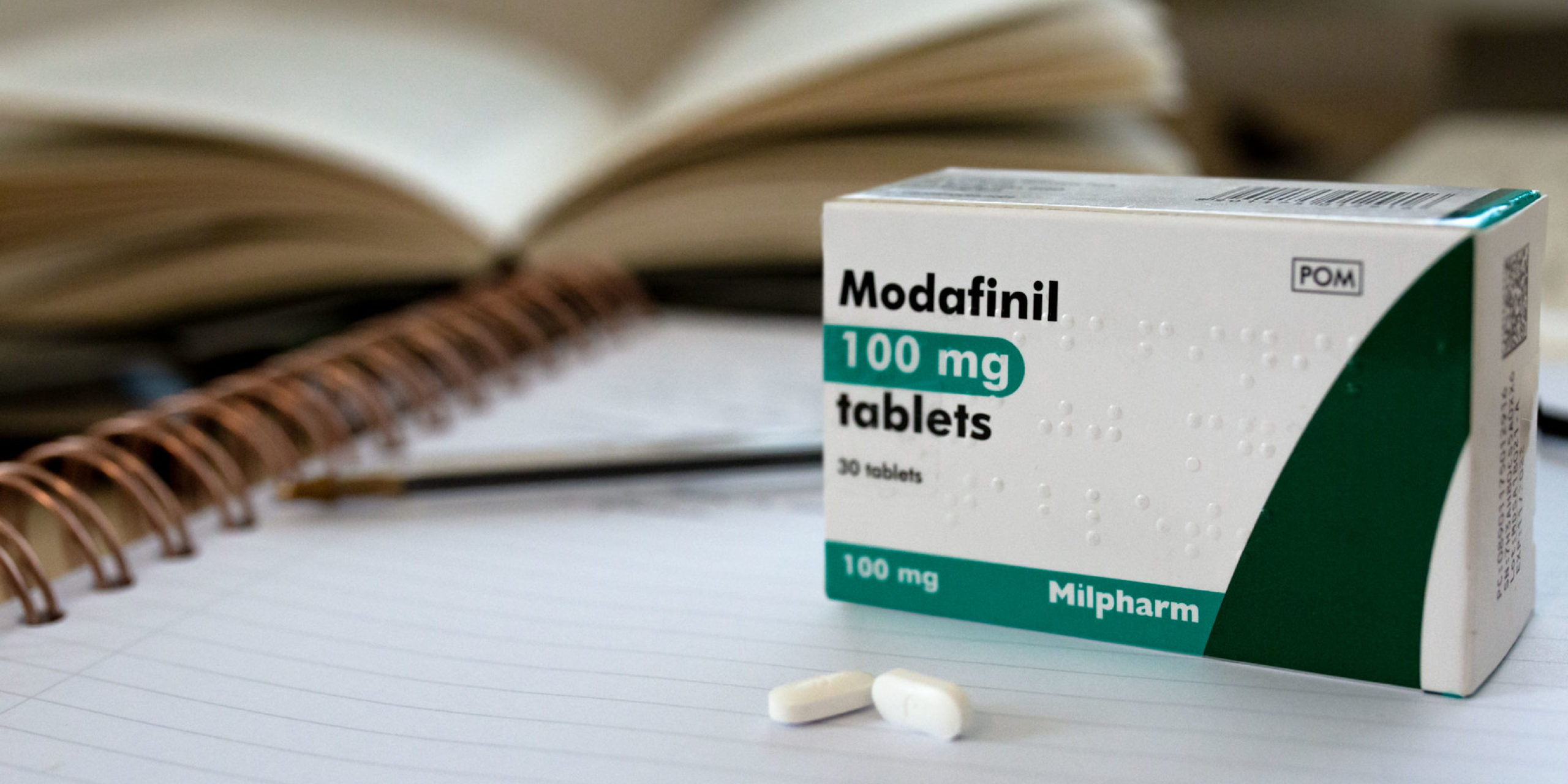
Limitations of Use In OSA, modafinil tablets are indicated to take care of extreme sleepiness instead of as cure for the fundamental obstruction. If steady good airway strain (CPAP) would be the treatment of option for a patient, a maximal exertion to treat with CPAP for an suitable timeframe ought to be created previous to initiating and during remedy with modafinil tablets for too much sleepiness.
[242] The scientific trials that have examined modafinil to be a therapy for stimulant abuse have did not reveal its efficacy as well as the optimum dose and period of modafinil treatment continue to be unclear, and modafinil isn't a encouraged remedy for stimulant abuse.[242] Schizophrenia[edit]
In applying the 6 criteria talked over over, FDA regarded irrespective of whether MRCs needs to be extra towards the 503A DDC Record and also to the 503B DDC Listing. FDA has tentatively concluded that MRCs satisfy the statutory criteria for inclusion on equally lists. As reviewed previously mentioned, MRCs are reliable oral dosage type drug items that include, or are meant to encompass, a drug-made up of Main enclosed inside a polymeric coating to launch an Lively component at specified charges, designs, or onsets throughout the GI tract to create systemic, enteric, or community action. The complexities linked to the manufacture of MRCs make a Begin Printed Web page 19783 heightened hazard that compounded items wouldn't produce the Energetic component as supposed, which might current a security issue to people. FDA won't think an outsourcing facility's compliance with CGMP specifications would address the concerns described previously mentioned with regards to formulation complexity, drug shipping and delivery mechanism complexity, dosage form complexity, complexity of achieving or examining bioavailability, compounding course of action complexity, and complexity of physicochemical or analytical screening of your drug product or category of drug products. FDA's CGMP polices incorporate the bare minimum present-day good manufacturing exercise for solutions for use in, and also the amenities or controls to be used for, the manufacture, processing, packing, or Keeping of the drug to guarantee that such drug satisfies the necessities of the FD&C Work as to basic safety, and has the identification and power and satisfies the quality and purity properties that it purports or is represented to have (see 21 CFR 210.
Though modafinil is usually identified for being safe and considerable adverse results are unusual, such as in pediatric narcolepsy scenarios (snooze Diseases in children), There may be proof that prolonged-expression usage can lead to tolerance in certain individuals.
Acquire this medication by mouth with a glass of water. Stick to the directions over the prescription label. Just take your doses at common intervals. Usually do not get your drugs far more frequently than directed. Don't halt using apart from in your health practitioner's guidance.
There is a issue with data submitted for this ask for. Evaluate/update the information highlighted underneath and resubmit the form.
polymers) at temperatures above their glass changeover temperatures and/or melting temperatures inside of an extruder. The objective of the HME method is to improve the solubility of inadequately h2o-soluble medications by converting the formulation factors into an amorphous stage (not crystalline) product with uniform information. HME is actually a process by which warmth and shear are applied to soften a combination of API and inactive elements in just an extruder that may be then pushed via an orifice with the target of converting the ingredients into an amorphous stage content with uniform information, generally known as the “extrudate.” HMEs were evaluated using the six requirements that FDA proposes to implement to ascertain regardless of whether drug merchandise or groups of drug products and solutions existing difficulties for compounding under sections 503A and 503B with the FD&C Act explained in portion V.A. higher than. HMEs have complex formulations as the extrudate must continue being a steady and amorphous reliable Option of API inside a matrix through the entire shelf life of the final drug product in order to obtain suitable product performance.
A lot from the investigate on the get more info topic of modafinil have revealed that modafinil is properly tolerated11–13. Such as, 1 analyze was carried out on frustrated patients utilizing modafinil To ease indicators of melancholy, such as tiredness and sleepiness.
DA was recorded in sequential five-moment epochs, and for most recordings FSCV was done at the same time at independent CFMs implanted in dorsal and ventral striata of only one animal.
the place [DA]p is definitely the focus of DA release per stimulus pulse, which can be accustomed to index DA launch; k
Kovacs' operate concentrates on maximizing cognitive capabilities and Mind wellbeing as a result of innovative, efficient neuroactive compounds that conquer standard pharmacokinetic problems. His contributions are pivotal in advancing the comprehension and treatment method of neurological disorders.
Be sure to Observe that in the event you involve your title, contact facts, or other info that identifies you in your body of one's opinions, that details will be posted on .
[2] The initial lists, if finalized as proposed, would include three groups of drug products that current demonstrable issues for compounding underneath the two sections 503A and 503B of your FD&C Act and, for that reason, wouldn't qualify to the exemptions in possibly area. The proposed conditions and classes of drug products and solutions are described underneath. As discussed under, to determine irrespective of whether a drug solution or group of drug goods provides demonstrable challenges for compounding FDA could take into consideration the criteria With this proposed rule separately and collectively, and keep in mind the challenges and benefits to individuals on the compounded drug product or types of drug solutions. On top of that, FDA is proposing a few types of drug products which were being, independently of one another, evaluated by FDA and introduced into the PCAC to generally be bundled over the DDC Record for part 503A as well as DDC Record for part 503B with the FD&C Act. From the celebration of a remain or invalidation of any criterion or of any entry with a DDC List, those conditions and entries that keep on being in influence would Start out Printed Website page 19780 keep on to function sensibly [3] to progress the statutory aims. It truly is FDA's intent to protect Every of the standards and entries over the DDC Lists, if finalized, to your fullest doable extent, to help you advance the targets described in segment III.A. A. Conditions for Assessing Drug Goods or Types of Drug Solutions to the DDC Lists (Proposed § 216.25(a))
Persistent administration of modafinil can enrich the removing of CYP3A4substrates. Dose adjustments can be required for individuals staying addressed with these and equivalent drugs9.